An Insight into Cavitation Phenomena
We all must have heard about the term ‘Cavitation’ sometime, more certainly if we have dealt with pumps or similar fluid application and probably might have also seen some effects of ‘Cavitation’. Cavitation is very common term in engineering domain especially if we are dealing with fluids. So let us have a detailed insight into this ‘Cavitation’ phenomenon through this blog.

Cavitation
What is ‘Cavitation’…?
‘Cavitation’ is defined as the phenomenon of formation of vapor phase of a liquid when it is subjected to reduced pressures at constant ambient temperature. So basically cavitation is a boiling process in a liquid as a result of pressure reduction rather than heat addition.

Experiment for boiling of water at reduced pressure
So what exactly happens during ‘Cavitation’…?
Let us see what happens during cavitation by taking the simple example of water. We know water boils on addition of heat. So if we imagine atmospheric pressure of 101 kPa and we heat water to 100 deg C we are going to see boiling of water.
Now consider a condition where we are going to keep temperature constant, let us assume it as at 15.5 deg C and then we are going to reduce pressure. In this case also at specific low pressure value water does boil. In the condition where temperature is 15.5 deg C water will boil at 1.7 kPa. This is what happens during cavitation. Liquid boils by lowering pressure rather than increasing temperature or heat addition.
As a simple demo example we can take the case of boiling water in vacuum as shown in the above figure. When the air is pumped out of the small container, the pressure is reduced, resulting in lowering of the boiling point temperature and ultimately the water boils at room temperature. The boiling process can be observed by placing the container on the overhead projector. (This is part of very informative demo series by Dept. of Physics, Univ. of Illinois at Urbana-Champaign)
Why does liquid boil when pressure is lowered…?
So to answer this question we must understand three basic concepts. Those are:
- atmospheric pressure,
- vapor pressure and
- the relation between temperatures and vapor pressure.
First let us discuss about the atmospheric pressure. We know that earth is surrounded by a layer of gas. Why does this gas stay in contact with earth ? This is because it is held in contact by gravity. When we define pressure we say pressure is force per unit area. This layer of air above us on earth is exerting a pressure on the surface of planet and this pressure is about 760 mm column of mercury at sea level. We refer to this pressure as atmospheric pressure.
Now let us discuss about the concept of vapor pressure. All liquids exert a certain vapor pressure at any temperature. This is the pressure value at the instant at that temperature when the liquid molecules escape into vapor phase. The vapor pressure increases with temperature. This is because at higher temperature the molecules are moving faster and capable to overcome the attractive intermolecular forces that bind them. So boiling occurs when the vapor pressure reaches or exceeds the surrounding pressure from the atmosphere or the pressure due to whatever else is in vicinity of the liquid. Standard atmospheric pressure is 0.101325 MPa which is defined as 1 atmosphere. At this pressure water boils at approximately 100 deg C.
In other words, one can say that the vapor pressure of water at that temperature (100 deg C) is 1 atmosphere. When we increase pressure, temperature required is higher before the vapor pressure reaches the surrounding pressure. Due to this water under pressure boils at a higher temperature. When the surrounding pressure is lower, vapor pressure reaches that pressure at a lower temperature. Hence water can boil at lowered pressure instead of temperature increase. There is a strong relation between vapor pressure and temperature. For example if we consider water, the variation of vapor pressure is as shown below:
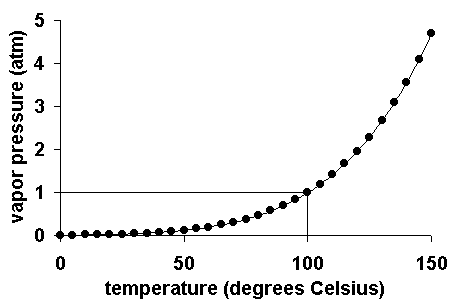
Vapor pressure-temperature diagram for water
At a higher temperature, more molecules have enough energy to escape from the liquid or solid. At a lower temperature, fewer molecules have sufficient energy to escape from the liquid or solid. Vapor pressure increases with increasing temperature.
Boiling and Cavitation… are they similar or different…?
The physics of cavitation inception is similar to boiling. The major difference between the two is the thermodynamic paths followed when vapor is formed. Boiling occurs when the local vapor pressure of the liquid rises above its local ambient pressure and sufficient energy is present to cause the phase change to a gas. Cavitation inception on the other hand occurs when the local pressure falls sufficiently far below the saturated vapor pressure.
What we see during ‘Cavitation’…?
During cavitation when the liquid encounters local regions of low pressure, the vapor bubbles start growing. These bubbles then flow along with the liquid and when they are in regions of higher pressure downstream, bubbles collapse on solid walls resulting in high local pressure.
How the word ‘Cavitation’ came into picture…?
The word ‘Cavitation’ comes from the Latin word “cavus” meaning hole or cavity. The vapor bubbles are like cavity in liquid flow.
Who discovered ‘Cavitation’…?

Cavitation, is a physics phenomenon occurring in nature. Osborne Reynolds was the scientist who first discovered cavitation. In 1894 he presented a short paper at a meeting of the British Association at Oxford. This paper was titled `Experiments concerned with the boiling of water in an open tube at ordinary temperatures’. The paper began with a description of the processes involved in the boiling of water by heating it. But it also highlighted the phenomenon of cavitation. An experiment was shown in the meeting. In the experiment a glass tube of internal diameter half an inch and length six inches was used. The tube had a neck in the middle, to less than a tenth of an inch bore. One end of the tube was coupled to water main. There was an inclination towards the open end downwards into a vessel filled with water. Then the water supply was turned on very slowly to fill the tube. As the velocity of flow was increased until a particular velocity a distinct sharp hiss was heard. Reynolds provided the following explanation for this phenomenon:
`As the bubbles of air and vapor would be carried with great velocity from the low pressure at the neck, where they formed, into the higher pressure in the wider portion of the expanding tube; so that the pressure being greater than the vapor tension, condensation would ensue and the bubbles would collapse…’. It is interesting to note that in the paper there is never a mention of the cavitation, but Oscar Reynolds was actually demonstrating the very understanding of it.
Is ‘Cavitation’ good or bad…?
The effects of ‘Cavitation’ phenomenon are sometimes a problem but as a concept it is also utilized for many positive outcomes. ‘Cavitation’ is a problem in processes like :
- In pump or fluid flow instruments ‘Cavitation’ has negative effects.
- Cavitation leads to noisy operation
- It can cause pitting, accelerated erosion and damage to components
- Cavitation can lead to imbalance and vibration which in turn damages components
- Overall effect of cavitation is loss of efficiency of equipments like pumps
Cavitation is also used for many significant purposes like :
- It is used in ultrasonic[1]
- It is used in particle breaking equipments
- It is used in water purification devices
- It is also used in treatment of kidney stones
Closer look at cavitation’ around hydrofoil…
Cavitation can occur in various situations and in various equipments and processes involving liquid. An interesting example to study cavitation is the cavitation around hydrofoil. Let us have a closer look at this study.
What is a hydrofoil ?
Hydrofoil is very similar to an airfoil. Foil has its meaning origin from the word wing. Hydrofoil is like a wing in liquid or water. The basic purpose of hydrofoils is to make the boat move faster. It can do this by taking hull out of the water. During normal operation of a boat, most of the energy is spend in moving the water in its path away. The hull does this job by pushing through the water in front of the boat. The hydrofoil basically reduces the energy spend in overcoming the drag on the hull by using the generated lift, to lift it above water.

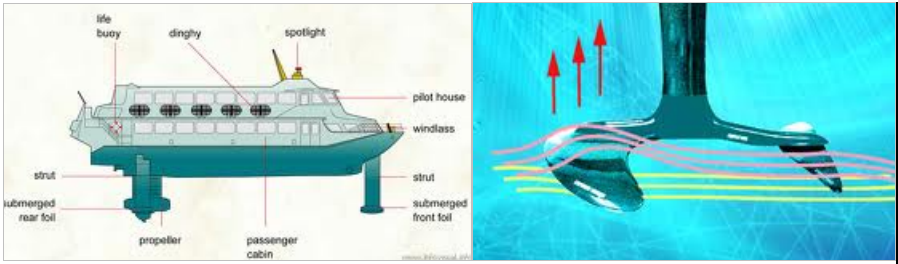
Hydrofoil working principle
How does a hydrofoil work…?
When the speed of the boat is low, the hull of the boat is in water and the hydrofoil which is below the boat is submerged in water. As the speed of boat increases there is lift created on the hydrofoil. This is similar to the lift created by wings on an airplane. At a certain critical speed the lift of hydrofoil will be same as the weight of boat and this will lift the boat up. Due to this, the hull of the boat comes above water. Now the boat has to counter the drag on the hydrofoil than the drag on the hull which is a much efficient way of cruising.
Let us see an interesting video from America’s Cup which shows the impact of hydrofoil.
http://www.youtube.com/watch?v=fnpwstBTOAI
‘Cavitation’ around a hydrofoil…
Many experiments have been performed to study cavitation around hydrofoils. These experiments are usually performed in circulating water channels. The velocity, pressure and temperature can be changed during experiments separately of each other. A symmetrical hydrofoil is then suspended in the channel with angle of attack so that lift is produced upwards. Now the ambient pressure is held constant at below atmospheric value and the flow velocity is increased till cavitation occurs. It is observed that cavitation first starts at the intersection of the strut and hydrofoil.

Initiation of cavitation
This is because, the presence of strut causes a pressure reduction compared to other regions of the hydroifoil. On the surface of the foil cavitation first occurs in the low pressure region of the laminar boundary layer separation. If the velocity is further increased cavitation begins near the leading edge. This is because as the velocity is high the Reynolds number is also high and transition from laminar to turbulent boundary occurs and a minimum pressure line is formed at the leading edge.

Cavitation near the leading edge
If the same phenomenon of cavitation is observed under stroboscopic light[2] it is observed that the cavitating region is actually made up of bubbles. Until the bubbles are in low pressure region, they grow. As they are swept along the flow to higher pressure region, the bubbles start collapsing near the trailing edge.
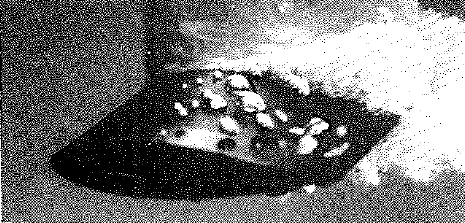
Cavitation under stroboscopic light
Let us see this phenomenon in detail in a short video.
http://www.youtube.com/watch?v=N7zcan3HToI
Coming up in next part…
This is the point where we end this Part 1 of An Insight into Cavitation blog. We will continue to study Cavitation in detail and well as its modeling methodologies further. Coming up in next blog we will try to cover below topics:
- Types of Cavitation
- Cavitation number
- Flow around propeller and Cavitation
- Effects of Cavitation
- Why and how noise is produce by Cavitation
In later parts we will also see how to model cavitation using CFD. So stay connected to this LearnCAx blog to get more insight into ‘Cavitation’!
Definitions :
- Ultrasonic – Ultrasonics is the application of ultrasound. Ultrasound can be used for medical imaging, detection, measurement and cleaning.
- Stroboscopic light – A strobe light or stroboscopic lamp, commonly called a strobe, is a device used to produce regular flashes of light. It is one of a number of devices that can be used as a stroboscope
References :
- Dept. of Physics, Univ. of Illinois at Urbana-Champaign Department of Chemistry, Purdue University
- Handbook of Fluid mechanics
- web.mit.edu
- Ocean Engineering Group, EWRE, Civil, Architectural and Environmental Engineering, UT Austin
- Marine Hydrodynamic Laboratory at MIT
- Hydrofoil ‘Cavitation’ Experiment at École Polytechnique Fédérale de Lausanne (EPFL)
- America’s Cup youtube channel
The Author
{module [317]}